Encouraging industry participation in the EU's Sixth Framework Programme: Issues, barriers and potential solutions
DOI:
https://doi.org/10.5912/jcb90Keywords:
research, 6FP, EU, industry participation, EU research associationsAbstract
There is around 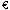 100m annually in the Sixth Framework Programme earmarked exclusively for small and medium-sized enterprises (SMEs) in the life sciences field. That is 15 per cent of the total amount available. But most of it has yet to be allocated. Why are biopharmaceutical SMEs not applying and how can they get access to this money?
100m annually in the Sixth Framework Programme earmarked exclusively for small and medium-sized enterprises (SMEs) in the life sciences field. That is 15 per cent of the total amount available. But most of it has yet to be allocated. Why are biopharmaceutical SMEs not applying and how can they get access to this money?
The Emerging Biopharmaceutical Enterprises (EBE) group teamed up with representatives of the European Commission for the 'Life Sciences, Genomics and Biotechnology for Health' Thematic Priority to help companies understand how they can benefit from the Programme and give them an opportunity to add their ideas to it. Attendees came from 60 biopharmaceutical companies, and the programme was structured to allow companies to ask questions, but also put forward ideas to be included in upcoming Calls for the Sixth Framework Programme, as well as suggestions for improving the Seventh Framework Programme to improve industry participation. The event also gave them the chance to ask the Commission representatives specific questions on the administrative aspects of the Programme. The event was organised in conjunction with the Scientific Officers responsible for the different areas in the Programme; and the external experts who advise the Commission on the content of the future calls and the overall strategy for priority areas and activities of research were also invited. This meeting was designed to be as relaxed and open as possible and put the companies directly in contact with the people who implement the Framework Programme. The input from this session based on the feedback of the attendees has been formally forwarded to the Commission and the Thematic Priority Advisory Group.
This paper discusses the aims and focus of the Sixth Framework Programme. It provides an overview of the discussion between the EBE and the representatives from the European Commission for the 'Life Sciences, Genomics and Biotechnology for Health' Thematic Priority and summarises the key problems and solutions arising therein.

